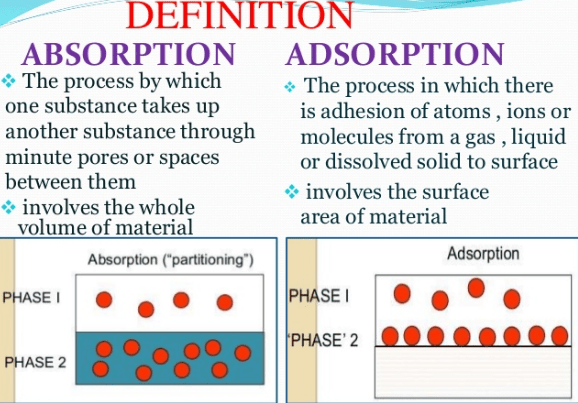
both adsorption and absorption are absorptions processes. when atoms are passes through some massive substance, it is called Absorption and adsorption occurs when the atomic particle totally diffuses or dissolves in an absorbent to make a solution which cannot be separated easily once dissolved. The difference between Absorption and Adsorption is that in Absorption, one substance is taken into the physical structure of the other substance and concern with the whole mass of the absorbent while in Adsorption, A substance or energy is atracted to a surface of another matter.it is a surface phenomenon.
In this post, you are going to learn about the Absorption and Adsorption by step with Diagrams.
- What is Absorption
- Examples and Applications
- What is Adsorption?
- Difference Table
- Lots more
So if you want to get benefits from this post you’ll love this post.
Let’s Dive right in..
An Overview
Adsorption is a process that accumulates only on the surface of substances such as liquids and gases. the particles or substances like liquids and gases are known as adsorbent. in adsorption, the substances do not enter into the adsorbent and remain only on the surface of concentration.
in contrast, absorption is a process in which the concentrations such as liquids and gases accumulate the concentration of absorbent and hence as a result substances distributed uniformly on the given surface that causes the increase in volume.
What is Absorption?
Absorption is a process in which a substance takes up another substance, such as a melting paper (solid) absorbing water (a liquid). Or a process in which a substance takes up radiation of a characteristic wavelength. The particles only accumulate on the surface.
Examples of absorbents:
Here are some of the most popular examples of absorbent materials.
- Silica Gel
- Graphite
- Alumina Gel
- Activated carbon
- Zeolites
What is Adsorption?
Adsorption is a process in which a substance or molecules adheres to the surface of another substance. Adsorption is important in some types of catalysis, notably where gases absorb on metal surfaces. The reaction is then made easier by a consequent lowering of activation.
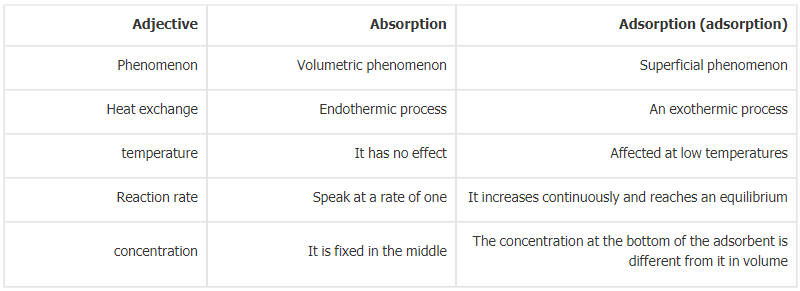
Difference between Absorption and Adsorption in Tabular Form | |
Absorption | Adsorption |
| Absorption is a situation in which any substance (atoms, ions, or molecules) is taken or absorbed by another substance, especially in a solid or liquid substance. | In adsorption, substances such as gas, liquids, or dissolved solids adhere to the surface of another material that can be solid or liquid. |
| It is known as a bulk phenomenon. | It is known as the Surface phenomenon. |
| The reaction occurs at a uniform rate. | The rate of the reaction increases slowly and achieves equilibrium. |
| Said to be an endothermic process. | Said to be an exothermic process. |
| The constant does not change throughout the medium. | The concentration changes from the bottom to the bottom of the absorbent. |
| There is no effect on temperature. | The adsorption works at a low temperature. |
| Cold storage, ice production, turbine inlet cooling, chillers are common examples of absorption. | Air conditioning, water purification, synthetic resin, and coolers are common examples of adsorption. |
| It remains throughout the process. | The concentration varies on the bulk of adsorbent. |
| The rate of absorption remains constant or uniform throughout the process. | The rate of adsorption is rapid at the initial stage. |
Related Topics: