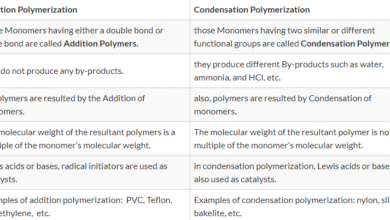
the major Difference Between Acid and Base is that an Acid is a substance that dissolved in water, ionizes and releases hydrogen ions in solution while the Base is a substance that dissolved in water, ionized and releases hydroxide or hydroxyl ions in solution.
Acid
- An acid is a chemical substance which donates proton during a chemical reaction.
- It turns blue litmus to red.
- Acids have sour taste. For example, unripe citrus fruits or lemon juice.
- They are corrosive in the concentrated form.
- Their aqueous solutions conduct electric current.
Base
- A base is a chemical substance which accepts a proton during a chemical reaction.
- It turns red litmus to blue.
- Bases have a bitter taste and feel slippery, for example, soap is slippery to touch.
- They are non-corrosive except the concentrated forms of NaOH and KOH.
- Their aqueous solutions conduct electric current.