what is an Alloy ? Metal or Nonmetal with examples
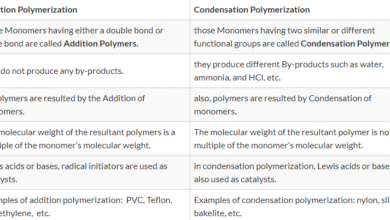
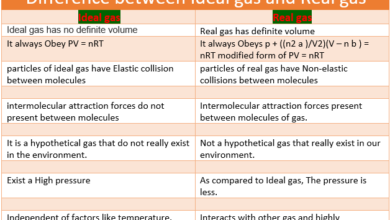
An alloy consists of two or more elements, at least one of which is a metal. It is formed by mixing the base metal with an alloying element to the required degree of strengths, malleability and other properties.
[wp_ad_camp_1]
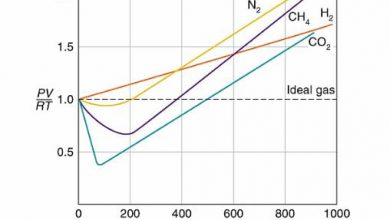
Alloy has many applications, from high strength and high resistance steel of light, magnesium-based alloy used in the construction of aircraft. In practice, very few metals are used in the pure state. Metals are characterized by their density, strength, thermal, and electrical conductivity and malleability (the easiness by which metals can be shaped into various structures).
Sometimes their qualities are not according to the requirements. Sometimes extraordinary strength, lightweight, and resistance to chemical action are required. No single metal can fulfill these requirements and thus alloys are used which express a combination of properties.